How Does The Amount Of Energy In Light Change As The Wavelength Increases
How can lite be used to make nutrient? When a person turns on a lamp, electrical energy becomes lite energy. Like all other forms of kinetic free energy, calorie-free can travel, change form, and be harnessed to do work. In the example of photosynthesis, light energy is converted into chemical free energy, which photoautotrophs use to build carbohydrate molecules. Still, autotrophs only use a few specific components of sunlight.
What Is Calorie-free Energy?
The sun emits an enormous amount of electromagnetic radiation (solar energy). Humans can come across only a fraction of this energy, which portion is therefore referred to as "visible light." The manner in which solar free energy travels is described equally waves. Scientists can make up one's mind the amount of energy of a wave by measuring its wavelength, the distance between consecutive points of a wave. A unmarried wave is measured from 2 consecutive points, such as from crest to crest or from trough to trough (Effigy ane).
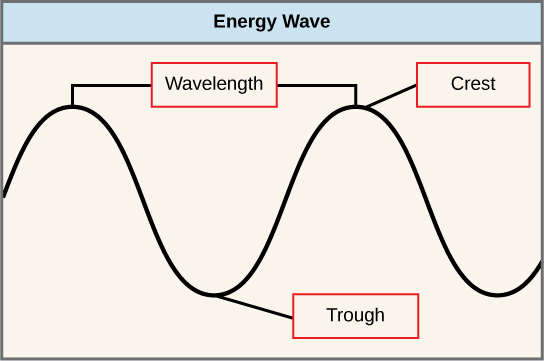
Effigy 1. The wavelength of a single wave is the distance between two consecutive points of similar position (two crests or two troughs) along the wave.
Visible light constitutes merely one of many types of electromagnetic radiation emitted from the sunday and other stars. Scientists differentiate the diverse types of radiant free energy from the lord's day within the electromagnetic spectrum. The electromagnetic spectrum is the range of all possible frequencies of radiation (Figure 2). The difference between wavelengths relates to the corporeality of energy carried by them.
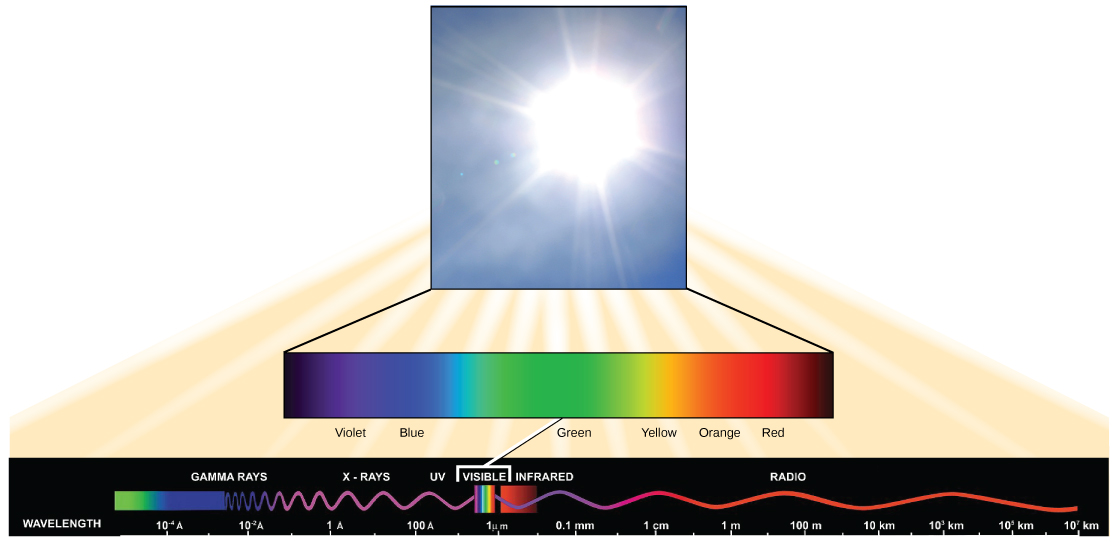
Figure 2. The sunday emits energy in the class of electromagnetic radiation. This radiations exists at different wavelengths, each of which has its own characteristic energy. All electromagnetic radiation, including visible light, is characterized by its wavelength.
Each type of electromagnetic radiation travels at a item wavelength. The longer the wavelength (or the more stretched out it appears in the diagram), the less energy is carried. Short, tight waves deport the about energy. This may seem casuistic, but think of it in terms of a piece of moving a heavy rope. It takes lilliputian attempt past a person to move a rope in long, broad waves. To make a rope motility in curt, tight waves, a person would need to apply significantly more than free energy.
The electromagnetic spectrum (Figure 2) shows several types of electromagnetic radiation originating from the sun, including 10-rays and ultraviolet (UV) rays. The higher-energy waves can penetrate tissues and damage cells and DNA, explaining why both Ten-rays and UV rays can be harmful to living organisms.
Absorption of Light
Lite energy initiates the process of photosynthesis when pigments blot the light. Organic pigments, whether in the homo retina or the chloroplast thylakoid, accept a narrow range of free energy levels that they can absorb. Energy levels lower than those represented past carmine low-cal are insufficient to raise an orbital electron to a populatable, excited (quantum) state. Energy levels college than those in blue light will physically tear the molecules apart, called bleaching. So retinal pigments tin simply "come across" (absorb) 700 nm to 400 nm light, which is therefore chosen visible light. For the aforementioned reasons, plants pigment molecules blot only light in the wavelength range of 700 nm to 400 nm; plant physiologists refer to this range for plants as photosynthetically active radiation.
The visible light seen by humans as white lite actually exists in a rainbow of colors. Certain objects, such as a prism or a drib of water, disperse white light to reveal the colors to the man middle. The visible low-cal portion of the electromagnetic spectrum shows the rainbow of colors, with violet and bluish having shorter wavelengths, and therefore higher energy. At the other end of the spectrum toward ruddy, the wavelengths are longer and accept lower energy (Figure 3).
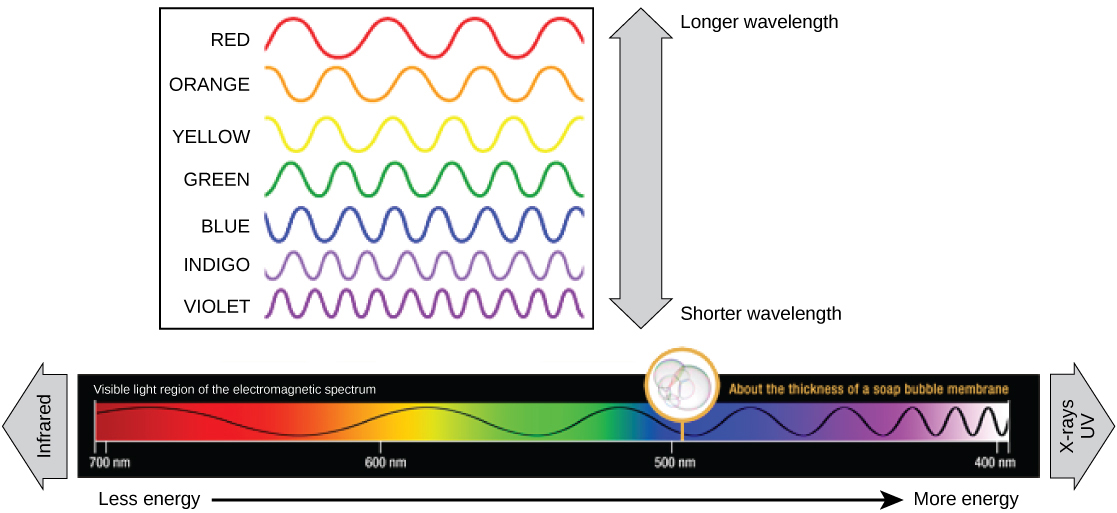
Figure 3. The colors of visible light do not carry the same corporeality of free energy. Violet has the shortest wavelength and therefore carries the almost energy, whereas ruby-red has the longest wavelength and carries the least amount of free energy. (credit: modification of piece of work by NASA)
Understanding Pigments
Different kinds of pigments exist, and each has evolved to absorb just certain wavelengths (colors) of visible light. Pigments reflect or transmit the wavelengths they cannot absorb, making them appear in the respective color.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments establish in plants and algae; each course has multiple types of pigment molecules. There are five major chlorophylls:a, b, c and d and a related molecule found in prokaryotes chosen bacteriochlorophyll. Chlorophyll a and chlorophyll b are found in higher constitute chloroplasts and will be the focus of the following discussion.
With dozens of different forms, carotenoids are a much larger grouping of pigments. The carotenoids plant in fruit—such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange skin (β-carotene)—are used as advertisements to attract seed dispersers. In photosynthesis,
carotenoids function equally photosynthetic pigments that are very efficient molecules for the disposal of backlog energy. When a leaf is exposed to full sun, the light-dependent reactions are required to process an enormous amount of energy; if that energy is non handled properly, it tin can practise significant damage. Therefore, many carotenoids reside in the thylakoid membrane, blot backlog free energy, and safely dissipate that energy every bit heat.
Each type of pigment tin exist identified by the specific pattern of wavelengths it absorbs from visible low-cal, which is theabsorption spectrum. The graph in Figure 4 shows the assimilation spectra for chlorophylla, chlorophyll b, and a type of carotenoid pigment chosen β-carotene (which absorbs blue and greenish light). Observe how each pigment has a singled-out set of peaks and troughs, revealing a highly specific pattern of absorption. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but non light-green. Because light-green is reflected or transmitted, chlorophyll appears green. Carotenoids blot in the short-wavelength blueish region, and reflect the longer yellow, ruddy, and orangish wavelengths.
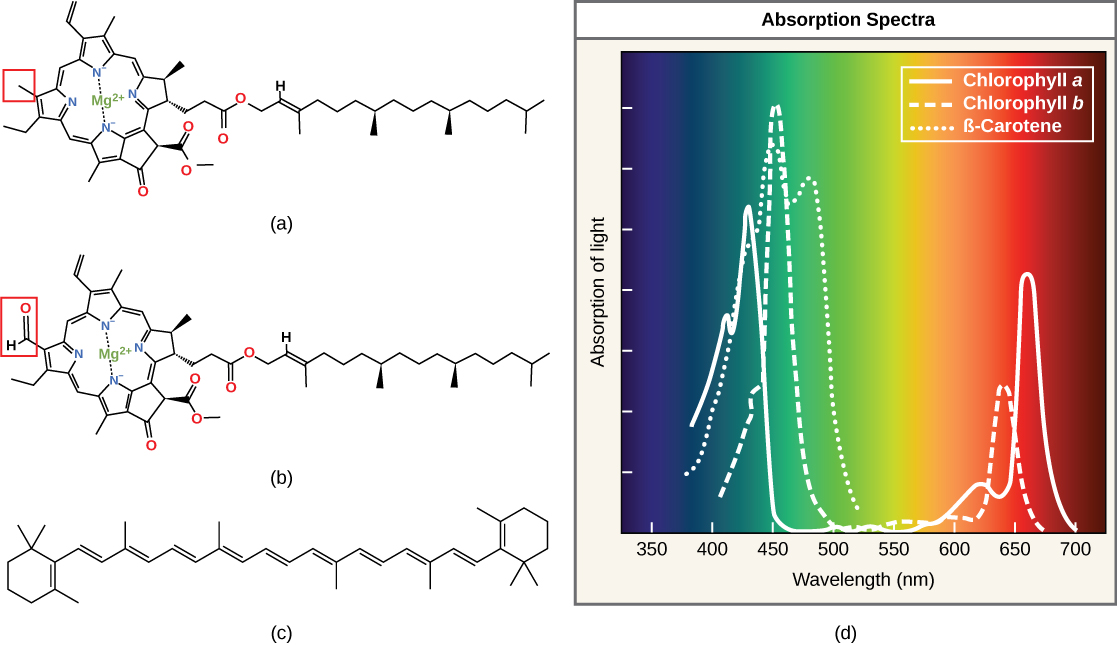
Figure four. (a) Chlorophyll a, (b) chlorophyll b, and (c) β-carotene are hydrophobic organic pigments found in the thylakoid membrane. Chlorophyll a and b, which are identical except for the part indicated in the red box, are responsible for the light-green color of leaves. β-carotene is responsible for the orange color in carrots. Each pigment has (d) a unique absorbance spectrum.

Figure 5. Plants that commonly grow in the shade have adjusted to low levels of calorie-free by irresolute the relative concentrations of their chlorophyll pigments. (credit: Jason Hollinger)
Many photosynthetic organisms take a mixture of pigments; using them, the organism can blot energy from a wider range of wavelengths. Non all photosynthetic organisms have full access to sunlight. Some organisms abound underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for low-cal. Plants on the rainforest floor must exist able to blot whatsoever bit of light that comes through, because the taller trees blot most of the sunlight and scatter the remaining solar radiations (Effigy 5).
When studying a photosynthetic organism, scientists can make up one's mind the types of pigments present past generating assimilation spectra. An musical instrument called aspectrophotometer can differentiate which wavelengths of light a substance can absorb. Spectrophotometers measure transmitted light and compute from it the absorption. Past extracting pigments from leaves and placing these samples into a spectrophotometer, scientists tin can identify which wavelengths of light an organism tin absorb. Boosted methods for the identification of plant pigments include diverse types of chromatography that dissever the pigments by their relative affinities to solid and mobile phases.
Source: https://courses.lumenlearning.com/ivytech-bio1-1/chapter/reading-spectrums-of-light/
Posted by: swihartthits1936.blogspot.com


0 Response to "How Does The Amount Of Energy In Light Change As The Wavelength Increases"
Post a Comment